SmartTrack™ aims to reduce the need for clinical studies in generic drug product approvals
AptarGroup, Inc. (NYSE: ATR), a global leader in drug and consumer product dosing, dispensing and protection technologies, today announces the commencement of a clinical study to validate its proprietary SmartTrack™ platform. The platform was developed by Aptar’s drug services company, Nanopharm. If validated, SmartTrack™ aims to reduce the need for clinical studies in generic drug product approvals by proving it can accurately predict clinical outcomes – removing a major barrier for pharma companies and regulators and paving the way for wider patient access to medications. This validation would establish SmartTrack™ as a credible in-vitro-in-silico alternative to comparative clinical endpoint (CCEP) studies, and a reliable approach to derisking in vitro-pharmacokinetic (PK) only approaches, specifically for generic inhaled drug products.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20250416330741/en/
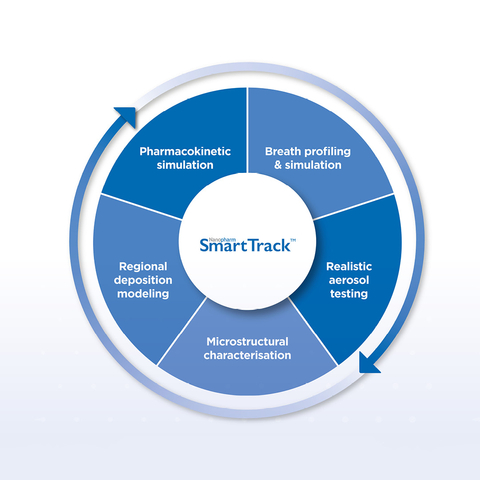
Nanopharm’s SmartTrack™ for Generic Inhalation Products
The study, which is expected to begin in Q2 2025, has been carefully designed based on detailed feedback from regulatory agencies to meet the requirements for obtaining a biowaiver of the CCEP for pharmaceutical companies working with Nanopharm as well as enabling the models to provide supportive data to the in vitro studies.
The study will involve radio-labelling three different commercially available pressurized metered-dose inhaler (pMDI) drug products and imaging regional lung deposition.
These results will be compared with regional deposition predictions from computational fluid dynamic (CFD) simulations conducted in collaboration with Fluidda, Medimprove and i2c Pharmaceutical Services.
Additionally, pharmacokinetic (PK) data from subjects will be collected to validate predictions from Nanopharm’s Simhalation™ platform, which uses physiologically based pharmacokinetic (PBPK) modeling.
The resulting study data will be submitted to the U.S. FDA as one of the first Model Master Files (MMF), which would allow multiple companies to benefit from this data when partnering with Aptar, similar to the well-established Drug Master File (DMF) route.
Gael Touya, President, Aptar Pharma, stated, “This clinical study marks a significant milestone in the evolution of Aptar’s offerings. Providing clinically validated data to our pharmaceutical partners and regulatory bodies will be crucial in demonstrating the platform’s viability, which could lead to accelerated approvals and broader access to generic inhaled medicines for more patients.”
In addition to supporting generic Abbreviated New Drug Application (ANDA)1 approvals, the study could also help accelerate and derisk other programs, such as the reformulation of pMDIs with new lower global warming potential (GWP) propellants, novel (drug product) combinations, developing drug products from other dosage forms, and New Chemical Entity (NCE) development into pMDIs.
The study is expected to conclude by the end of 2025. Interested parties are encouraged to contact Nanopharm to explore how this package can support their product development and approval strategies.
About Aptar
Aptar is a global leader in drug and consumer product dosing, dispensing and protection technologies. Aptar serves a number of attractive end markets including pharmaceutical, beauty, food, beverage, personal care and home care. Nanopharm is a leading provider of tailored analytical, modelling and pharmaceutical development services, with a focus on orally inhaled and nasal drug products (OINDP). The company’s unique analytical technologies and formulation development tools enable seamless translation of pre-clinical product development through to CMC, IVBE and cGMP manufacturing and release, whether for generic drug products or new molecular entities. This helps pharmaceutical companies to holistically understand how all properties of the combination drug products influence product functionality to accelerate and derisk product development in the niche field of OINDP. For more information, visit www.nanopharm.co.uk and www.aptar.com.
This press release contains forward-looking statements, including the potential outcomes of the SmartTrack™ technology. Forward-looking statements generally can be identified by the fact that they do not relate strictly to historical or current facts and by use of words such as “expects,” “anticipates,” “believes,” “estimates,” “future,” “potential,” “continues” and other similar expressions or future or conditional verbs such as “will,” “should,” “would” and “could” are intended to identify such forward-looking statements. Forward-looking statements are made pursuant to the safe harbor provisions of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934 and are based on our beliefs as well as assumptions made by and information currently available to us. Accordingly, our actual results or other events may differ materially from those expressed or implied in such forward-looking statements due to known or unknown risks and uncertainties that exist in our operations and business environment including, but not limited to: the successful integration of acquisitions; the regulatory environment; and competition, including technological advances. For additional information on these and other risks and uncertainties, please see our filings with the Securities and Exchange Commission, including the discussion under “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in our Form 10-K and Forms 10-Q. We undertake no obligation to update publicly any forward-looking statements, whether as a result of new information, future events or otherwise, except as otherwise required by law.
View source version on businesswire.com: https://www.businesswire.com/news/home/20250416330741/en/
Contacts
Aptar Investor Relations Contact:
Mary Skafidas
mary.skafidas@aptar.com
+1 347 351 6407
Aptar Pharma Media Contact:
Ciara Jackson
ciara.jackson@aptar.com
+49 151 1951 6502




