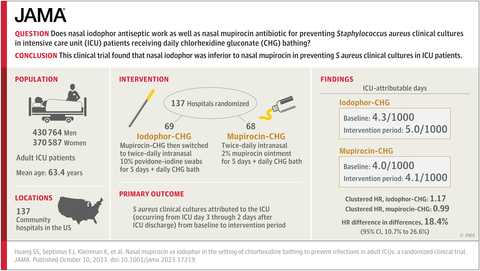
Mupirocin outperformed nasal antiseptic solution against Staphylococcus infections in study of 137 hospitals
A large multi-state study by the Centers for Disease Control and Prevention, Harvard Pilgrim Health Care Institute, HCA Healthcare and the University of California, Irvine has found that a nasal antibiotic ointment, mupirocin, which is currently used daily for intensive care unit (ICU) patients in only one-third of U.S. hospitals, is highly effective at preventing Staphylococcus aureus infections in critically ill patients, outperforming an antiseptic solution.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20231010228617/en/
(Photo: Business Wire)
This study involved more than 800,000 ICU patients in 137 HCA Healthcare hospitals, and built upon an earlier study among these research partners demonstrating that daily bathing with antiseptic chlorhexidine soap plus nasal mupirocin for all ICU patients prevented many bloodstream and other serious infections. This strategy is called “decolonization” because it reduces the amount of bacteria on the body in order to reduce infection risk. Today, as a result of the previous study, the majority of ICUs bathe patients with chlorhexidine; however, only one-third of hospital ICUs provide nasal mupirocin to all patients, largely due to fears of fueling antibiotic resistance.
Because of a concern that disease-causing bacteria might become resistant to mupirocin, the investigators compared mupirocin to an alternative nasal antiseptic product, known as povidone-iodine or iodophor.
The Mupirocin-Iodophor Swap Out Trial directly compared nasal mupirocin to nasal iodophor in the context of chlorhexidine bathing. The trial found that mupirocin worked significantly better than the antiseptic in preventing Staphylococcus aureus infection, including those due to methicillin-resistant S. aureus, or MRSA. The CDC-funded study was published today in the Journal of the American Medical Association (JAMA).
“This study further supports CDC guidance on using a strategy that combines nasal decolonization plus CHG bathing in ICU patients. Furthermore, the data show that using mupirocin for nasal decolonization may be preferred over iodophor because it is more effective at preventing S. aureus infections or colonization. S.aureus infections account for nearly a quarter of the infections in ICUs in the United States,” said John Jernigan, MD, Branch Chief, CDC’s Epidemiology, Research and Innovations Branch.
The results resolved the question about whether nasal treatment is necessary in addition to chlorhexidine bathing to prevent these ICU infections. “This large study confirms that clearing the nose of bacteria prevents infection, and that the choice of product matters. Mupirocin antibiotic ointment remains the best treatment, and serious ICU infections can be avoided by simply giving patients mupirocin for the first five days of an ICU stay along with daily chlorhexidine bathing. Povidone-iodine does not work as well,” remarked lead investigator, Susan S. Huang, M.D., M.P.H., professor in the Division of Infectious Diseases at the University of California, Irvine School of Medicine.
The 137 participating community hospitals spanned 18 states and are part of HCA Healthcare, a leader in pragmatic research in real-life settings. Half of the hospitals continued their standard practice of treating ICU patients with mupirocin nasal ointment, and the other half switched to nasal povidone-iodine. All hospitals used the nasal product for five days plus chlorhexidine for daily bathing.
Importantly, this trial showed a durable benefit from mupirocin. The use of an antibiotic ointment for the past 10 years at HCA Healthcare did not diminish its impact. There was similar persistent clinical benefit even after nearly a decade of continuous ICU use, suggesting that use of chlorhexidine soap and mupirocin had not lost their effect. This is important because widespread antibiotic use can lead to antibiotic resistance in some instances.
Because of the size of the study and the fact that it was conducted at such a wide range of community hospitals, the results are generally believed to be applicable to hospitals across the country.
“HCA Healthcare is honored to continue collaborating with the CDC, UCI Health and the Harvard Pilgrim Health Care Institute to leverage our scale to answer clinical questions that will benefit patients everywhere,” said Kenneth Sands, MD MPH, chief epidemiologist at HCA Healthcare. “The remarkable benefit from our earlier REDUCE MRSA study to evaluate this protocol for preventing ICU infections led to widespread adoption across our hospital system 10 years ago. This new trial confirms the effectiveness of the mupirocin CHG protocol, and we have already put this science into practice, establishing universal use of mupirocin as the preferred agent for nasal decolonization in all hospitals.”
The study was conducted through a longstanding scientific consortium, including HCA Healthcare, Harvard Medical School’s Department of Population Medicine at the Harvard Pilgrim Health Care Institute, the University of California, Irvine and the Centers for Disease Control and Prevention. This same scientific group conducted the REDUCE MRSA Trial over 10 years ago, which first showed that this decolonization regimen could reduce MRSA by 37% and bloodstream infections by 44%. Now, the Mupirocin-Iodophor Swap Out Trial showed that switching from mupirocin to iodophor produced 18% less protection from S. aureus infections.
“A unique opportunity presented itself to compare our REDUCE MRSA trial from 10 years ago to the Mupirocin-Iodophor Swap Out Trial. Although mupirocin was clearly better than povidone-iodine, there was also a strong suggestion that povidone-iodine was also preventative when compared to ICUs in the prior trial that received no nasal anti-infective. This provides even more evidence for the important role of the nose as a source of infection,” adds senior author Richard Platt, MD, MSc, professor and chair of the Department of Population Medicine at the Harvard Pilgrim Health Care Institute and Harvard Medical School.
The Mupirocin-Iodophor Swap Out Trial adds to a growing set of evidence that reducing the amount of bacteria on the skin and in the nose through decolonization can protect patients from infection during high-risk moments. This same scientific group not only conducted the REDUCE MRSA Trial, but also the ABATE Infection Trial which showed that decolonization of hospitalized patients with medical devices outside of the ICU reduces both bloodstream infections and antibiotic-resistant pathogens.
“This study provides additional evidence of the benefit of reducing the burden of pathogens in or on a patient’s body,” said Dr. Jernigan. “Not only does this reduce risk for patients who are carrying harmful germs, but it may also reduce the spread of these germs to others. To maximize the impact of this promising strategy, we hope the Mupirocin-Iodophor Swap Out Trial will inspire the research and development of a greater number of agents that can be brought to market to extend the benefit of pathogen reduction.”
Additional information about the Mupirocin-Iodophor Swap Out Trial can be found in the following JAMA author interview podcast: https://edhub.ama-assn.org/jn-learning/audio-player/10.1001/jama.2023.19978.
About Centers for Disease Control and Prevention
The Centers for Disease Control and Prevention (CDC) is one of the major operating components of the Department of Health and Human Services. CDC works 24/7 protecting America’s health, safety and security. Whether disease start at home or abroad, are curable or preventable, chronic or acute, or from human activity or deliberate attack, CDC responds to America’s most pressing health threats. CDC is headquartered in Atlanta and has experts located throughout the United States and the world.
About Harvard Pilgrim Health Care Institute’s Department of Population Medicine
The Harvard Pilgrim Health Care Institute's Department of Population Medicine is a unique collaboration between Harvard Pilgrim Health Care and Harvard Medical School. Created in 1992, it is the first appointing medical school department in the United States based in a health plan. The Institute focuses on improving health care delivery and population health through innovative research and education, in partnership with health plans, delivery systems, and public health agencies. Point32Health is the parent company of Harvard Pilgrim Health Care and Tufts Health Plan. Follow us on Twitter and LinkedIn.
About HCA Healthcare
Nashville-based HCA Healthcare is one of the nation’s leading providers of healthcare services comprising 182 hospitals and approximately 2,300 ambulatory sites of care, including surgery centers, freestanding ERs, urgent care centers, and physician clinics, in 20 states and the United Kingdom. With its founding in 1968, HCA Healthcare created a new model for hospital care in the United States, using combined resources to strengthen hospitals, deliver patient-focused care and improve the practice of medicine. With a robust system for analyzing clinical data across large and diverse patient populations, HCA Healthcare is a leader in pragmatic research like the Swap Out, ABATE and REDUCE MRSA trials that can help identify new standards of care. HCA Healthcare is a learning health system that uses its more than 37 million annual patient encounters to advance science, improve patient care and save lives.
About UCI Health
UCI Health is the clinical enterprise of the University of California, Irvine, and the only academic health system in Orange County. Patients can access UCI Health at primary and specialty care offices across Orange County and at its main campus, UCI Medical Center in Orange, Calif. The 459-bed, acute care hospital, listed among America’s Best Hospitals by U.S. News & World Report for 22 consecutive years, provides tertiary and quaternary care, ambulatory and specialty medical clinics, behavioral health and rehabilitation services. UCI Medical Center is home to Orange County’s only National Cancer Institute-designated comprehensive cancer center, high-risk perinatal/neonatal program and American College of Surgeons-verified Level I adult and Level II pediatric trauma center and regional burn center. UCI Health serves a region of nearly 4 million people in Orange County, western Riverside County and southeast Los Angeles County. Follow us on Facebook, Instagram, LinkedIn and Twitter.
View source version on businesswire.com: https://www.businesswire.com/news/home/20231010228617/en/
Contacts
HCA HEALTHCARE:
Investor Contact
Frank Morgan
615-344-2688
Media Contact
Harlow Sumerford
615-344-1851
UCI HEALTH:
Sophia Papa
661-369-6968
spapa@hs.uci.edu
CENTERS FOR DISEASE CONTROL AND PREVENTION:
Martha Sharan
404-998-1787
msharan@cdc.gov
HARVARD PILGRIM HEALTH CARE INSTITUTE:
Maya Dutta-Linn
Maya_Dutta-Linn@hphci.harvard.edu
Jessica Meuleman
Jessica_Meuleman@hphci.harvard.edu




