
GLP-1 (Semaglutide) drugs like Ozempic and Wegovy produced by Novo Nordisk A/S (NYSE: NVO) and Mounjaro and Zepbound (Tirzepatide) produced by Eli Lilly and Co. (NYSE: LLY) have taken the mainstream by storm. These drugs have transformed the medical sector. The Oprah Winfrey special, "Shame, Blame and the Weight Loss Revolution," brought more attention to the benefits of clinical weight-loss treatments as she calls obesity a disease. Obesity can increase the risk of over 200 diseases. The popularity of GLP-1 drugs has caused NYSE: NVO">a gold rush for biotechs looking to cash in the NYSE: NVO">growing demand affecting nearly 60% of the world's population.
Viking Therapeutics Inc. (NASDAQ: VKTX) has one of the most promising drugs yet to come to market. The company specializes in treating metabolic and endocrine disorders. Viking's lead drug candidate is VK2735. Like Mounjaro, VK2735 is a dual agonist for both the GLP-1 hormone and glucose-dependent insulinotropic polypeptide (GIP). Its clinical trials are going so well that many brokers have come out ahead of FDA approval submissions and given the stock $100+ price targets. Oppenheimer raised its price target to $138. Viking has a pipeline of drugs that also address diseases associated with obesity, like VK2809, a treatment for non-alcoholic steatohepatitis (NASH) and fatty liver. It attempts to reduce the fat in the liver to prevent it from causing inflammation and damage, leading to cirrhosis and potentially death.
The Faulty Body Mass Index Categorization
The body mass index (BMI) is used to define overweight people when it climbs over 25, while obese people are defined by a BMI over 30. Nearly 60% of the population falls into the overweight to obese category. However, critics argue that BMI only measures weight and height without considering overall body composition and shape. The BMI would have classified Arnold Schwarzenegger and all heavyweight bodybuilders as obese individuals.
The Triple Threat
Current GLP-1 medications are once-a-week injectable treatments with side effects ranging from nausea, vomiting, and diarrhea to gastrointestinal pain. Mounjaro had more clinical trial participants quit due to gastrointestinal side effects than Ozempic. Most users quit taking GLP-1 drugs after a year due to painful side effects.
Viking's therapy has shown more tolerability to side effects, which may not be as severe. Its clinical trials have also shown weight loss comparable to or even greater than Ozempic and Zepbound.
VK2735 Injectable Phase 1 Clinical Trials
The VK2735 Phase I clinical trials were successful and met safety and tolerability endpoints, as not a single patient dropped out of the two studies for adverse side effects. This is a significant advantage that neither Ozempic nor Mounjaro can claim. VK2735 injectable clinical trials were performed using a single-ascending dose (SAD) and multiple-ascending dose (MAD) study for obese patients with a BMI of 30 or above. Average body weight reduction from baseline was up to 7.8% after 21 days. All treatment-emerged adverse events were found to be tolerable with mild to moderate severity with no serious adverse events.
Phase 2 trials
The Phase 2 VENTURE trials comprised 176 adult participants with raised doses. Primary and secondary endpoints were all met successfully. The treatments were safe and well tolerated, with the majority of side effects being categorized as mild and moderate. Patients lost up to 14.7% of baseline body weight after 13 weeks, with significant differences between the placebo. At least 88% of participants lost at least 10% weight loss versus 4% for placebo.
As for safety and tolerability, 13% discontinued from the study versus 14% in the placebo and 92% of the participants reported mild or moderate severity of side effects. GI-related adverse effects were found early in the study and decreased with frequency. The weekly rate of nausea did not exceed 5% at any point after the first week. Viking plans to meet with the FDA to discuss the next steps toward the application for approval. Check out the sector heatmap on MarketBeat.
Positive Phase 1 Oral Tablet Trial Results
An oral table version of VK2735 completed its Phase 1 clinical trials with positive results. The 28-day MAD study demonstrated mean body weight reductions from baseline up to 5.3 and relative to placebo at 3.3%.Up to 57% of participants achieved at least 5% weight loss compared to 0% for placebo. Treatment duration beyond 28 days would likely provide more weight loss, which will be performed in Phase 2.
Viking Therapeutics CEO Bian Lin, Ph.D., commented, "We are particularly pleased with the initial safety and tolerability data, which suggest a differentiated profile with minimal gastrointestinal-related side effects. We believe that an oral agent with good tolerability could represent an attractive potential treatment option for patients with obesity. We look forward to observing a longer treatment window and potential higher dose in an upcoming Phase 2 trial."
Viking Therapeutics analyst ratings and price targets are at MarketBeat. Viking Therapeutics peers and competitor stocks can be found with the MarketBeat stock screener.
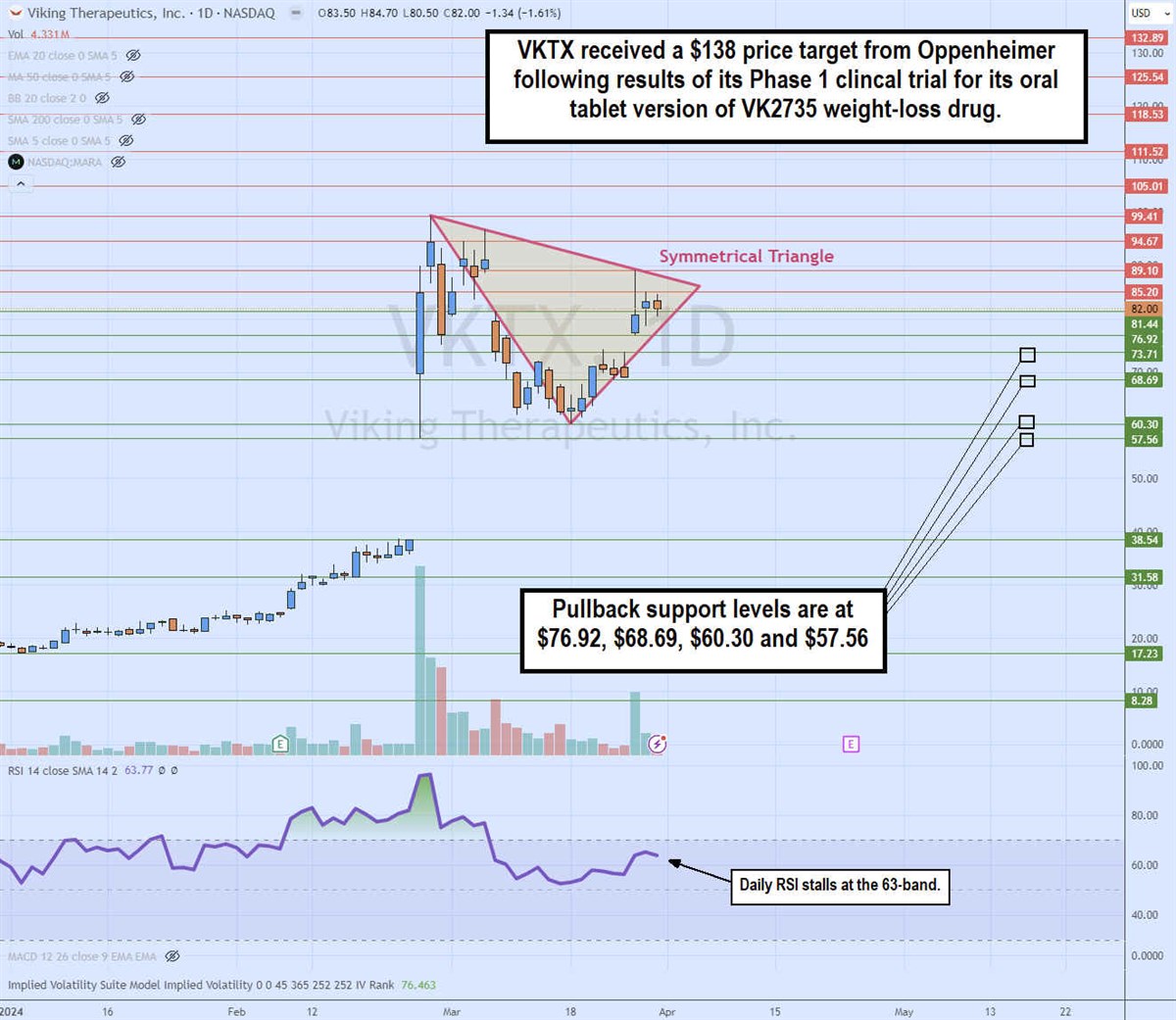
Daily Symmetrical Triangle
The daily candlestick chart for VKTX illustrates a daily symmetrical triangle pattern. It could even be considered a pennant since the flagpole on the gap is apparent. The descending trendline formed off the $99.41 high on February 28, 2024. The ascending lower trendline formed at the $60.34 swing low on March 18, 2024. The trendlines converge at the apex point at which a breakout through the descending trendline triggers a breakout, or a breakdown through the ascending trendline will trigger a breakdown. The daily relative strength index (RSI) is stalling at the 63-band, ready to bounce through the 70-band or retreat. Pullback support levels are at $76.92, $68.69, $60.30 and $57.56.




