-Super Speed of Only Nine Months
GERMANTOWN, MD / ACCESSWIRE / August 19, 2024 / uBriGene Biosciences (uBriGene), a global CDMO leader, is excited to announce that its strategic partner, InnoVec Biotherapeutics, has received FDA clearance for its Investigational New Drug (IND) application for IVB103, an AAV-based gene therapy for neovascular age-related macular degeneration (nAMD). This crucial nod from the FDA marks the official launch of IVB103 into the clinical trial phase for treating nAMD.
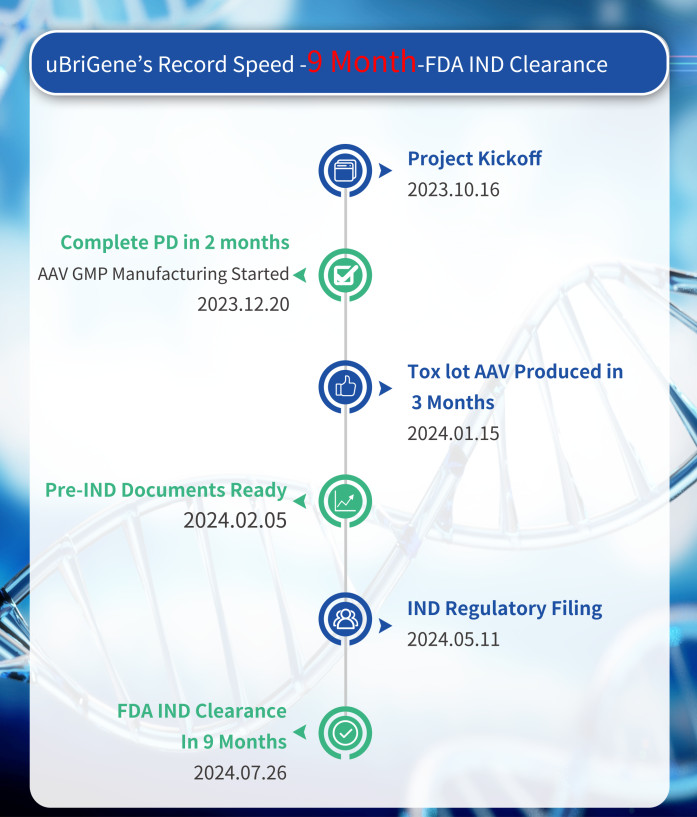
"As the CDMO partner for InnoVec's IVB103 project, we are incredibly proud to have played a key role in achieving this milestone at an incredible speed," said Dr. Xiulian Sun, cofounder and CTO of uBriGene. "Our team's dedication and expertise have enabled us to provide end-to-end CDMO services, including plasmid process development and GMP manufacturing, AAV custom capsid process development, method validation, AAV GMP manufacturing, in-house QC release testing, stability studies, and IND regulatory documentation. We look forward to continuing our collaboration with InnoVec Biotherapeutics to accelerate this innovative therapy for patients in need."
Dr. Cheng Wang, CEO of InnoVec Biotherapeutics, expressed his enthusiasm: "Receiving FDA clearance for our IND application is a significant step forward for IVB103 and for patients suffering from nAMD. uBriGene's unwavering support and exceptional capabilities have been instrumental in our journey. Their extensive process development and manufacturing expertise and regulatory know-how have made it possible to get the IND clearance in just nine months. We are excited to advance IVB103 into clinical trials and are grateful for uBriGene's invaluable partnership."
IVB103, developed by InnoVec Biotherapeutics, is poised to be a game-changer in treating nAMD. This novel drug leverages custom capsids for intravitreal administration, developed via InnoVec's EASI-FIND® AAV discovery platform. The FDA clearance signifies the safety and efficacy of InnoVec's custom capsids for intravitreal administration, as well as the compliance of uBriGene's manufacturing processes.
Since the project's official kick-off on October 16, 2023, uBriGene has closely aligned with InnoVec's needs, ensuring seamless communication and coordination across production and regulatory strategies. uBriGene is dedicated to supporting cell and gene therapy projects with its remarkable ‘uBriGene speed,' aiming to expedite the journey of new therapies to clinics.
About InnoVec Biotherapeutics
InnoVec Pharmaceutical Technology Co., Ltd. (InnoVec Biotherapeutics) is an innovative clinical-stage gene therapy company, driven by its ground-breaking custom AAV engineering platform for enhanced Human tissue delivery. InnoVec focuses on developing cutting-edge gene therapy technologies and drug solutions. Their preclinical pipelines span ocular neuronal and muscle areas, with a commitment to creating effective gene delivery tools for a wide range of diseases.
About uBriGene Biosciences
Founded in 2015, uBriGene Biosciences is a leading Contract Development and Manufacturing Organization for advanced therapeutic medicinal products (ATMPs). The company provides integrated CDMO and CRO solutions, encompassing services for cell therapy products, viral vectors, and RNA-related products, with in-house QC testing and regulatory IND filing. Our GMP-validated Maryland facility offers one-stop CDMO services from process development to manufacturing, driving global advancements in ATMPs.
For more information please contact:
uBriGene Biosciences
Alex Chen
President of NA Operations
800-663-2528
contact@ubrigene.com
Contact Information
Alex Chen
President of NA Operations
contact@ubrigene.com
800 663 2528
SOURCE: uBriGene Biosciences Inc.
View the original press release on newswire.com.




